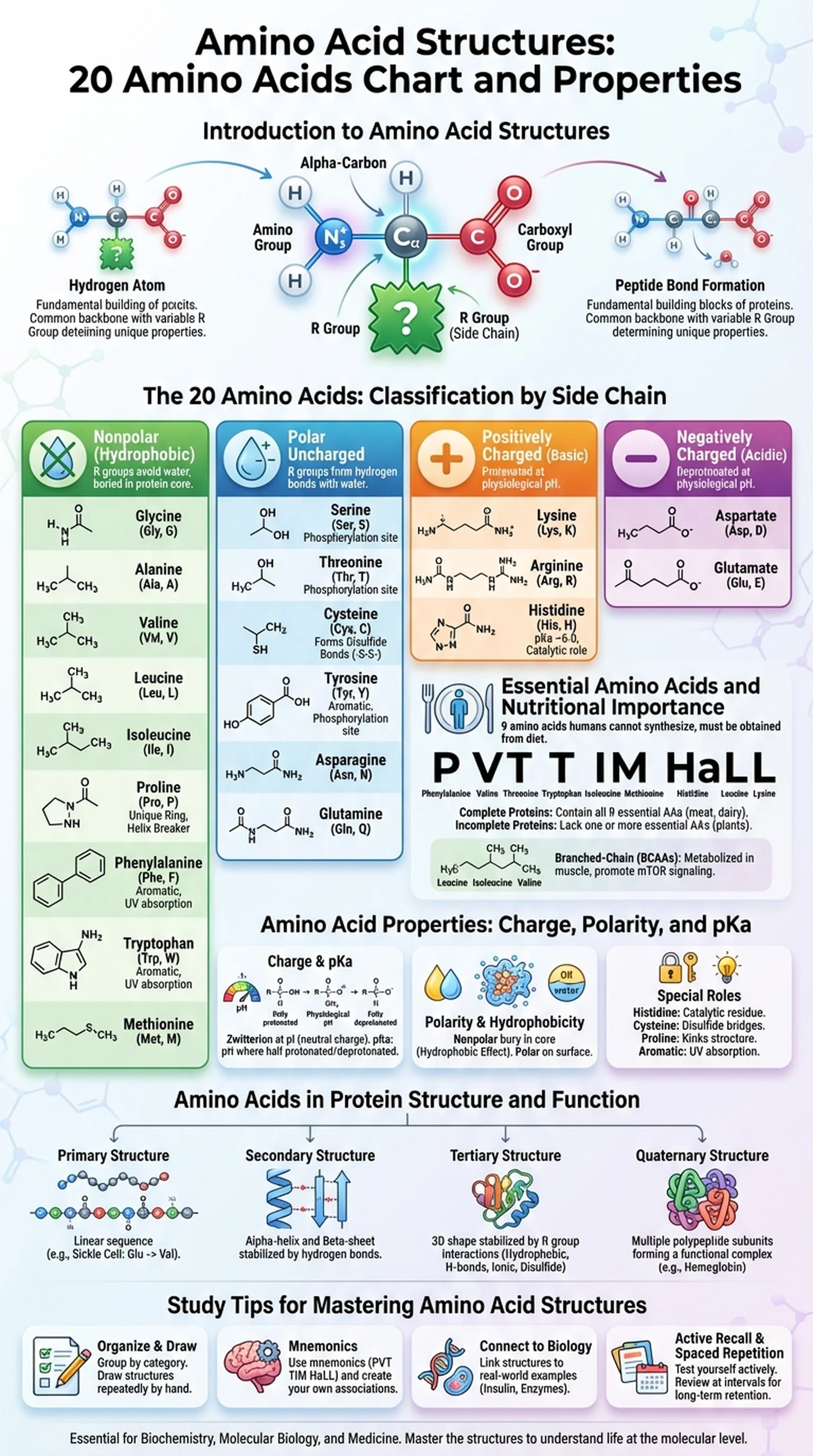
Introduction to Amino Acid Structures
Amino acid structures form the molecular alphabet of life, encoding the information needed to build every protein in the human body. There are 20 amino acids that are genetically encoded and incorporated into proteins during translation. Each amino acid shares a common backbone consisting of a central alpha-carbon bonded to an amino group (-NH2), a carboxyl group (-COOH), a hydrogen atom, and a unique side chain (R group). It is the side chain that gives each amino acid its distinctive chemical personality and determines how it contributes to protein folding, function, and interactions.
Understanding amino acid structures is foundational for biochemistry, molecular biology, and medicine. When students encounter an amino acid chart for the first time, the diversity of R groups can seem overwhelming. However, the 20 amino acids can be organized into logical categories based on side chain properties: nonpolar (hydrophobic), polar uncharged, positively charged (basic), and negatively charged (acidic). This classification system transforms a seemingly random list into a structured framework that is far easier to learn and apply.
The importance of amino acid properties extends well beyond memorization for exams. Mutations that swap one amino acid for another can dramatically alter protein function, as seen in diseases like sickle cell anemia where a single glutamate-to-valine substitution changes hemoglobin's behavior. Drug design frequently targets specific amino acid residues in enzyme active sites. Clinical laboratory tests measure levels of particular amino acids to diagnose metabolic disorders. In short, a thorough knowledge of the 20 amino acids and their structural features is indispensable for any student pursuing a career in the biomedical sciences.
Key Terms
The molecular arrangements of the 20 genetically encoded amino acids, each consisting of a common backbone and a unique R group (side chain).
The variable chemical group attached to the alpha-carbon that distinguishes one amino acid from another and determines its chemical properties.
The central carbon atom of an amino acid to which the amino group, carboxyl group, hydrogen, and R group are all attached.
A covalent bond formed between the carboxyl group of one amino acid and the amino group of another during protein synthesis.