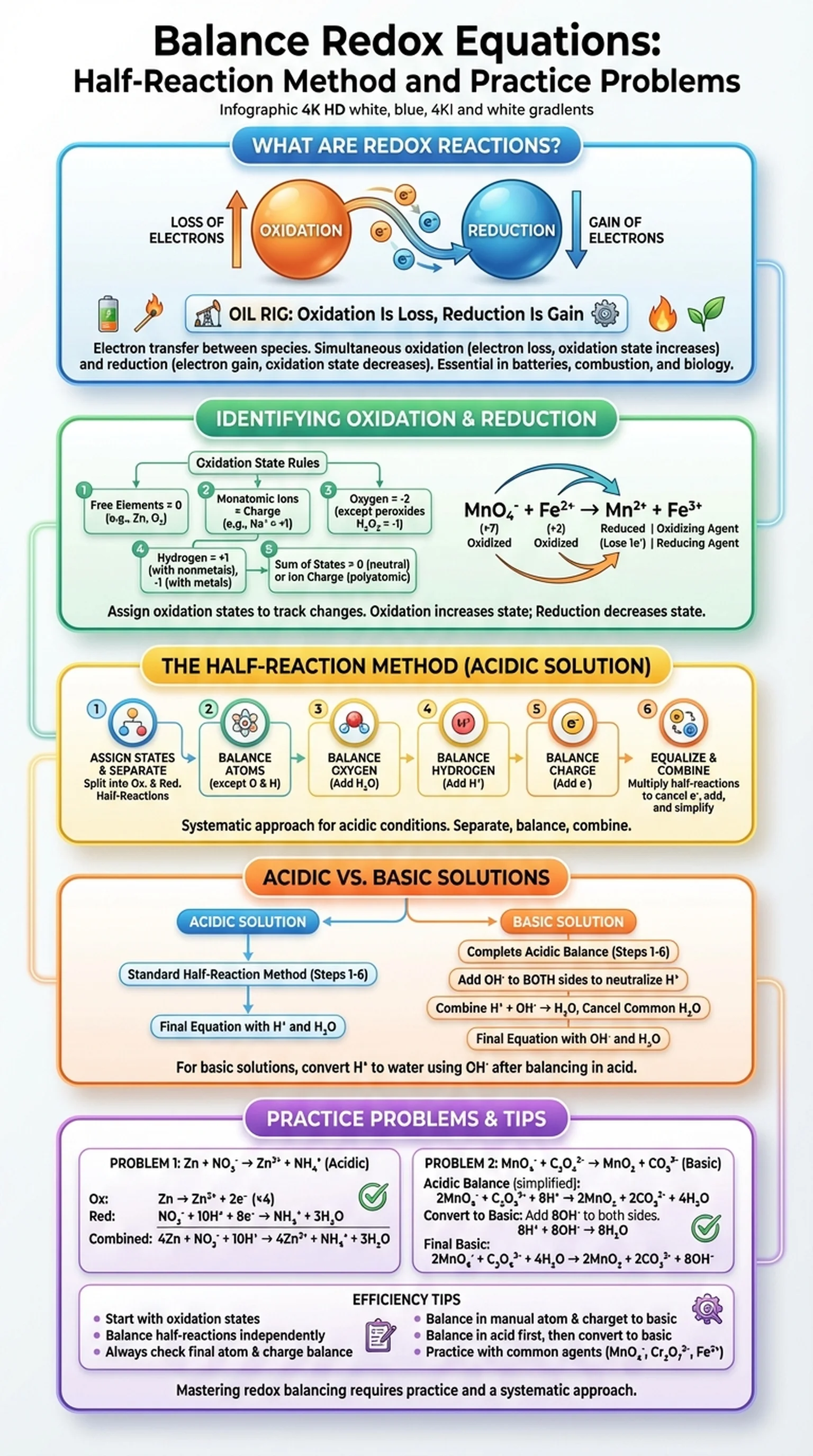
What Are Redox Reactions?
Redox reactions, short for oxidation-reduction reactions, are chemical processes in which electrons are transferred between species. These reactions are among the most important in all of chemistry, governing everything from cellular respiration and photosynthesis to the corrosion of metals and the operation of batteries. Understanding how to balance redox equations is an essential skill for students in general chemistry, organic chemistry, biochemistry, and electrochemistry.
In every redox reaction, two complementary processes occur simultaneously. Oxidation is the loss of electrons by a species, while reduction is the gain of electrons. These processes are always coupled — when one substance is oxidized, another must be reduced. A helpful mnemonic is "OIL RIG": Oxidation Is Loss, Reduction Is Gain. The substance that is oxidized serves as the reducing agent because it donates electrons, while the substance that is reduced serves as the oxidizing agent because it accepts electrons.
Redox reactions can be identified by tracking changes in oxidation states. The oxidation state of an atom is a bookkeeping tool that assigns a hypothetical charge based on a set of rules. When an atom's oxidation state increases, it has been oxidized. When it decreases, it has been reduced. For example, when iron reacts with oxygen to form rust (Fe2O3), iron's oxidation state increases from 0 to +3 (oxidation) while oxygen's decreases from 0 to -2 (reduction).
Learning to balance a redox equation properly is critical because these reactions involve not just conservation of atoms, but also conservation of charge. Simple inspection methods that work for non-redox reactions are often insufficient for redox reactions, which is why specialized techniques such as the half-reaction method are essential. In the sections that follow, we will explore how to identify oxidation and reduction, master the half-reaction method for balancing redox equations, and work through practice problems in both acidic and basic solutions.
Key Terms
A chemical reaction involving the transfer of electrons between species, consisting of simultaneous oxidation and reduction.
The loss of electrons by a species, resulting in an increase in oxidation state.
The gain of electrons by a species, resulting in a decrease in oxidation state.
The substance that is reduced in a redox reaction, accepting electrons from the reducing agent.
The substance that is oxidized in a redox reaction, donating electrons to the oxidizing agent.