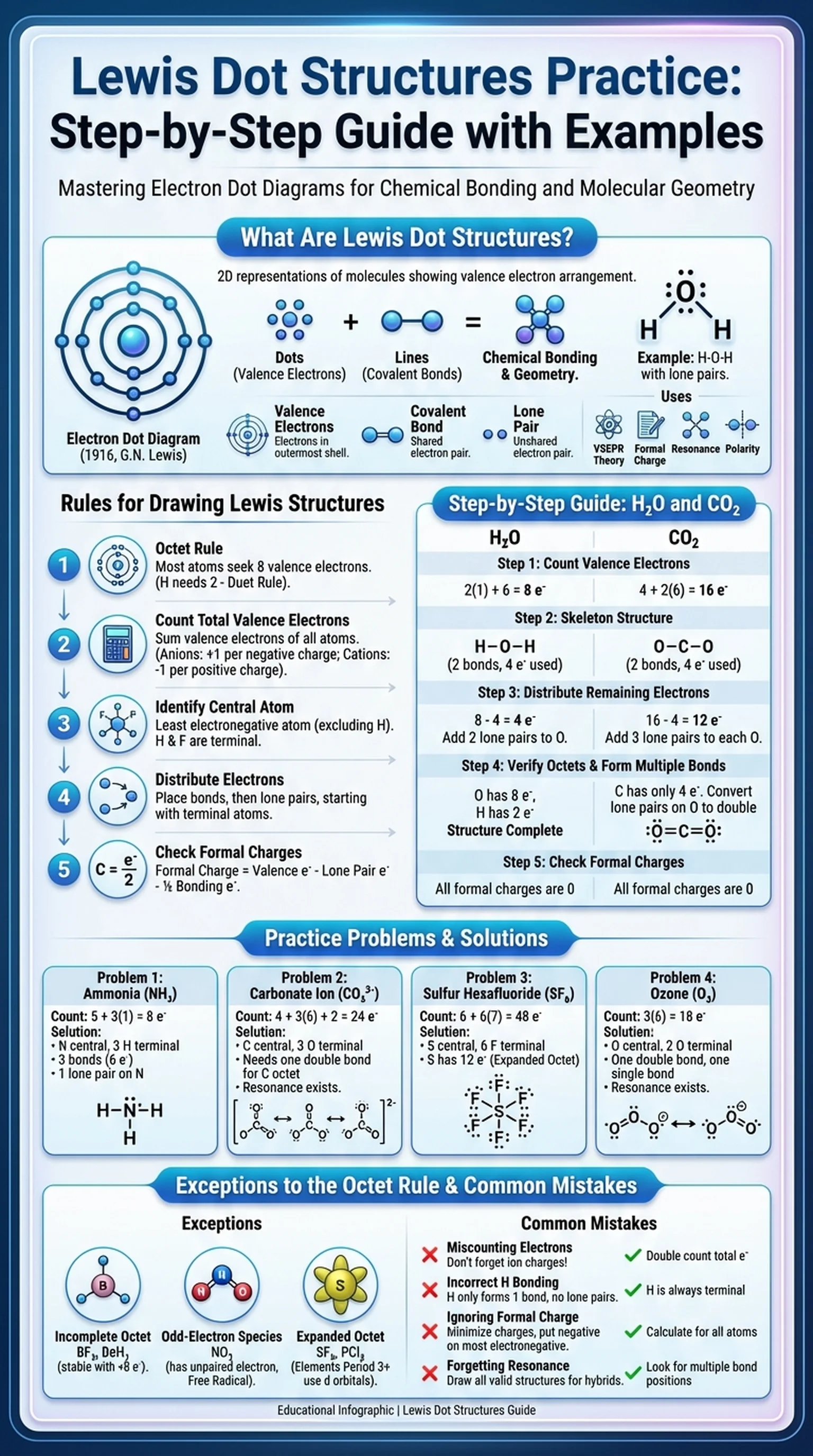
What Are Lewis Dot Structures?
Lewis dot structures, also known as electron dot diagrams, are two-dimensional representations of molecules that show the arrangement of valence electrons around atoms. Developed by American chemist Gilbert N. Lewis in 1916, these diagrams remain one of the most fundamental tools in chemistry for visualizing chemical bonding and molecular geometry. Whether you are taking introductory chemistry, organic chemistry, or preparing for standardized exams, lewis dot structures practice is essential for building a strong foundation in chemical reasoning.
A lewis dot structure uses dots to represent valence electrons and lines to represent covalent bonds between atoms. Each line corresponds to a shared pair of electrons, while unshared pairs (lone pairs) are shown as pairs of dots on individual atoms. The goal of a lewis structure is to show how atoms in a molecule share or transfer electrons to achieve a stable electron configuration, typically an octet for main-group elements.
Lewis structures provide critical information that extends beyond simple bonding patterns. They allow chemists to predict molecular geometry using VSEPR theory, determine formal charges on individual atoms, identify resonance structures, and assess whether a molecule is polar or nonpolar. For students preparing for the AP Chemistry exam, the MCAT, or general chemistry finals, fluency in drawing and interpreting lewis structures is non-negotiable.
The power of the electron dot diagram lies in its simplicity. With just a periodic table and knowledge of a few rules, you can represent the bonding in molecules ranging from simple diatomic gases like H2 and O2 to complex polyatomic ions like sulfate and phosphate. In the following sections, we will cover the rules for drawing lewis structures, walk through step-by-step examples, and provide lewis dot structures practice problems to solidify your skills.
Key Terms
A diagram that shows the arrangement of valence electrons around atoms in a molecule using dots for lone pairs and lines for bonds.
The electrons in the outermost shell of an atom that participate in chemical bonding.
Another name for a Lewis dot structure, emphasizing the use of dots to represent valence electrons.
A pair of valence electrons that is not shared between atoms and remains on a single atom.
A chemical bond formed by the sharing of one or more pairs of electrons between two atoms.