What Is Glycolysis?
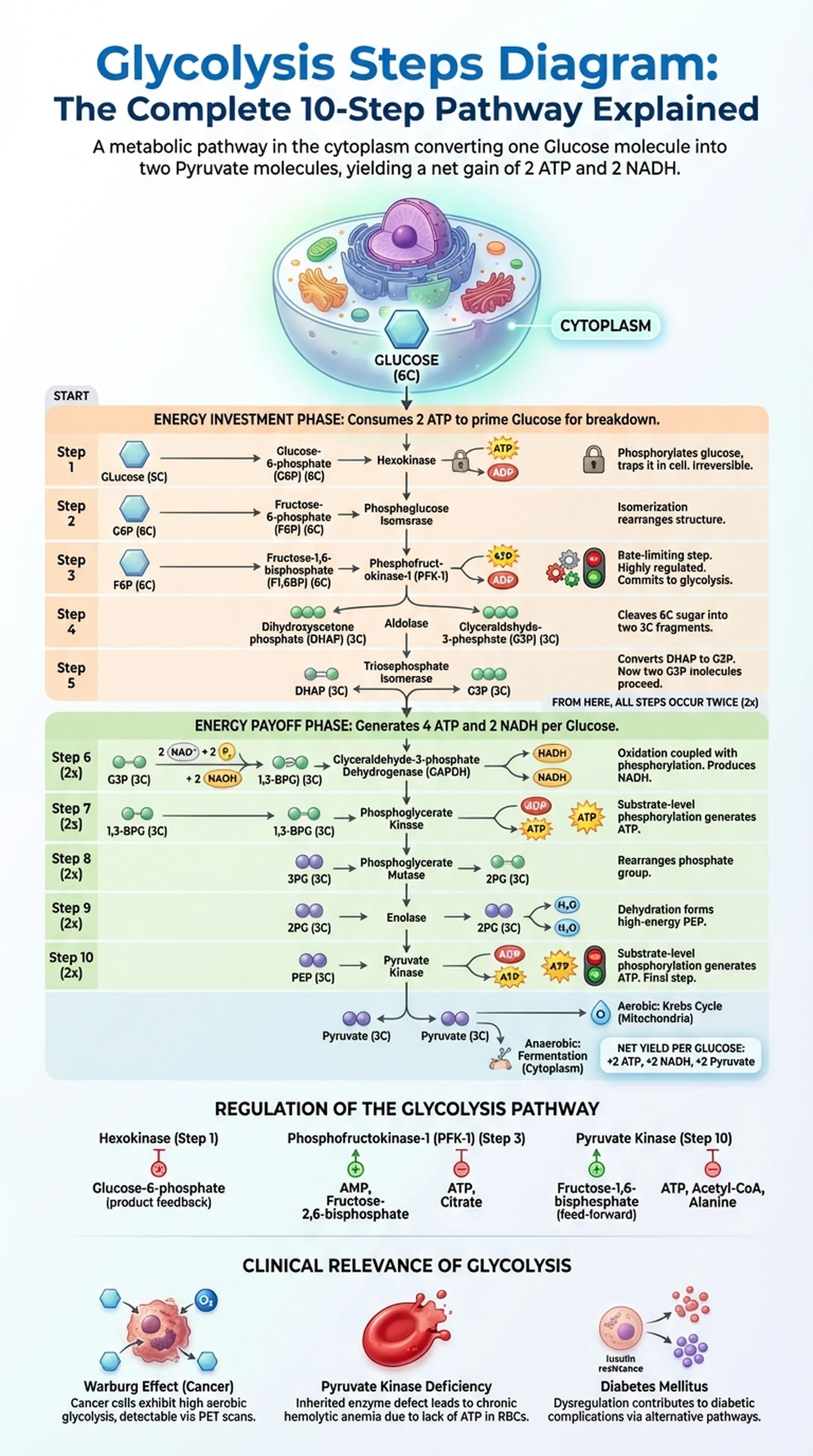
Glycolysis is the metabolic pathway that converts one molecule of glucose into two molecules of pyruvate, generating a net gain of two ATP and two NADH in the process. The word glycolysis comes from the Greek words for "sweet" (glykys) and "splitting" (lysis), reflecting the pathway's fundamental action of splitting a six-carbon sugar into two three-carbon fragments. Glycolysis is the first stage of both aerobic and anaerobic cellular respiration and takes place in the cytoplasm of virtually all living cells.
The glycolysis pathway is remarkable for its universality. From bacteria to human beings, nearly every organism on Earth uses glycolysis to extract energy from glucose. This universality suggests that the glycolysis process evolved very early in the history of life, likely before the atmosphere contained significant amounts of oxygen. Because glycolysis does not require oxygen, it can operate under both aerobic and anaerobic conditions, making it the primary energy-producing pathway for organisms living in oxygen-poor environments.
For students of biochemistry and cell biology, understanding the glycolysis steps diagram is a foundational skill. The pathway consists of 10 sequential enzymatic reactions, each catalyzed by a specific enzyme. These reactions can be divided into two phases: the energy investment phase (steps 1 through 5), which consumes ATP, and the energy payoff phase (steps 6 through 10), which generates ATP and NADH. A well-labeled glycolysis steps diagram is an invaluable study tool because it allows you to visualize the flow of carbon, energy, and electrons through the entire glycolysis process at a glance.
Key Terms
A metabolic pathway in the cytoplasm that breaks down one glucose molecule into two pyruvate molecules, yielding a net gain of 2 ATP and 2 NADH.
The complete sequence of 10 enzymatic reactions that constitute glycolysis, from glucose to pyruvate.
The overall biochemical process of splitting glucose into pyruvate, consisting of energy investment and energy payoff phases.
A three-carbon molecule produced as the end product of glycolysis; it can enter the Krebs cycle (aerobic) or undergo fermentation (anaerobic).
The gel-like substance within the cell membrane where glycolysis occurs, outside the mitochondria.