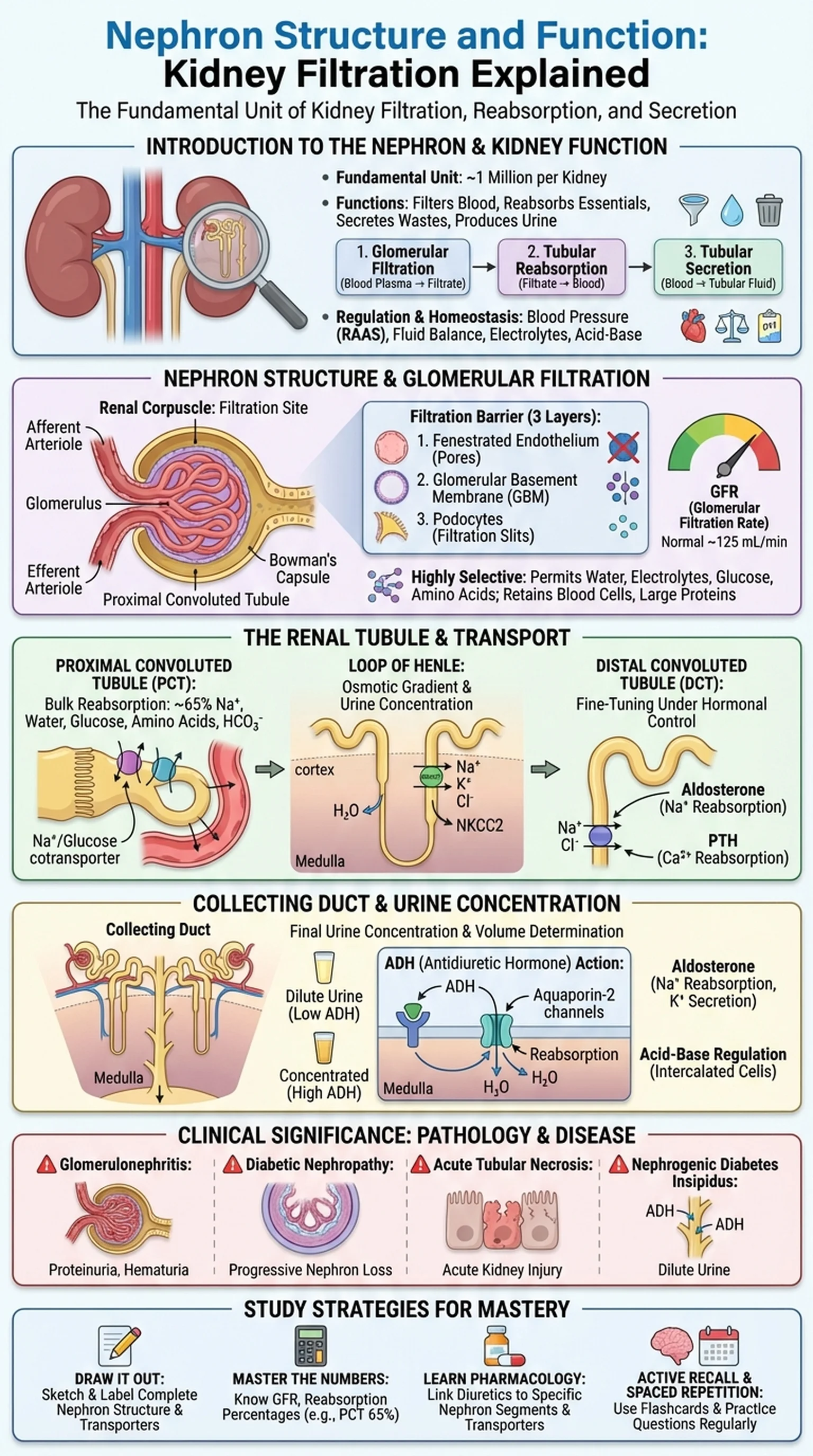
Introduction to the Nephron and Kidney Function
The nephron is the fundamental structural and functional unit of the kidney. Each human kidney contains approximately one million nephrons, and together they perform the critical tasks of filtering blood, reabsorbing essential substances, secreting waste products, and producing urine. Understanding nephron structure and nephron function is foundational for students of anatomy, physiology, and medicine because the kidneys regulate fluid balance, electrolyte concentrations, blood pressure, acid-base homeostasis, and the excretion of metabolic waste.
Kidney function depends on three sequential processes carried out by the nephron: glomerular filtration, tubular reabsorption, and tubular secretion. Glomerular filtration is the first step, in which blood plasma is filtered through the glomerular capillaries into Bowman's capsule. Tubular reabsorption recovers water, glucose, amino acids, and ions from the filtrate back into the peritubular capillaries. Tubular secretion adds additional waste products and excess ions from the blood into the tubular fluid. The final product, urine, represents only about 1% of the original filtrate volume, demonstrating the extraordinary efficiency of the nephron.
Renal physiology encompasses the study of how these processes are regulated to maintain homeostasis under varying conditions. When blood pressure drops, the kidneys activate the renin-angiotensin-aldosterone system (RAAS) to conserve sodium and water. When blood pressure rises, natriuretic peptides promote sodium and water excretion. The nephron is therefore not merely a passive filter but an active, hormonally responsive organ that adjusts its function moment by moment. A thorough understanding of nephron structure provides the anatomical framework necessary for comprehending these dynamic physiological and pathological processes.
Key Terms
The fundamental structural and functional unit of the kidney, responsible for filtering blood, reabsorbing essential substances, secreting wastes, and producing urine.
The collective physiological activities of the kidneys, including filtration, reabsorption, secretion, electrolyte balance, blood pressure regulation, and acid-base homeostasis.
The branch of physiology concerned with the function of the kidneys, including glomerular filtration, tubular transport, urine concentration, and hormonal regulation.
The process by which blood plasma is filtered through the glomerular capillaries and Bowman's capsule, producing a protein-free filtrate that enters the renal tubules.