What Are Functional Groups in Organic Chemistry?
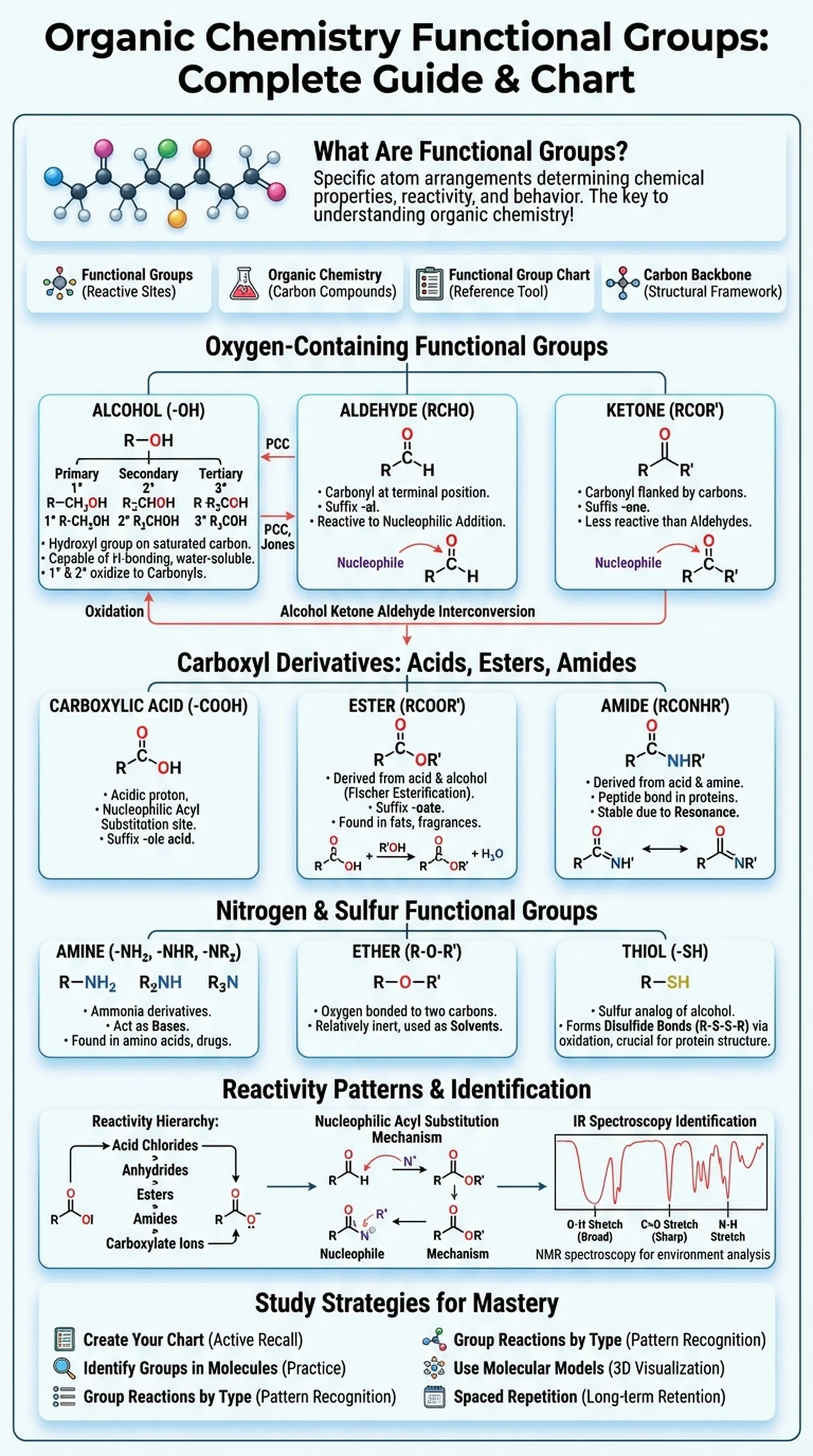
Functional groups are specific arrangements of atoms within organic molecules that determine the chemical properties, reactivity, and behavior of the compound. In organic chemistry, the carbon backbone of a molecule provides its structural framework, but it is the functional groups attached to that backbone that dictate how the molecule will react with other substances. Mastering functional groups is therefore the single most important step in understanding organic chemistry as a whole.
A functional group chart is an essential study tool that organizes the major organic functional groups by structure, naming conventions, and characteristic reactions. The most commonly encountered functional groups include hydroxyl groups (alcohols), carbonyl groups (aldehydes and ketones), carboxyl groups (carboxylic acids), amino groups (amines), ester linkages, ether linkages, and various others. Each group has a distinctive bonding pattern: for example, an alcohol features an -OH group bonded to a saturated carbon, while an aldehyde contains a carbonyl group (C=O) at the terminal position of a carbon chain.
The importance of functional groups extends far beyond the classroom. In biochemistry, the functional groups present on amino acids determine protein folding and enzyme activity. In pharmacology, modifying a single functional group on a drug molecule can dramatically alter its potency, selectivity, and metabolic fate. In materials science, the functional groups on polymer chains determine physical properties like solubility and melting point. For students preparing for the MCAT, DAT, or organic chemistry coursework, building a strong foundation in organic functional groups is the key that unlocks the logic of reaction mechanisms, synthesis planning, and molecular recognition.
Key Terms
Specific arrangements of atoms within organic molecules that determine chemical reactivity and properties, such as hydroxyl, carbonyl, carboxyl, and amino groups.
The branch of chemistry focused on the structure, properties, and reactions of carbon-containing compounds and their functional groups.
A reference table organizing the major organic functional groups by structure, nomenclature, and characteristic properties for study and identification.
The chain or ring of carbon atoms that forms the structural skeleton of an organic molecule, to which functional groups are attached.