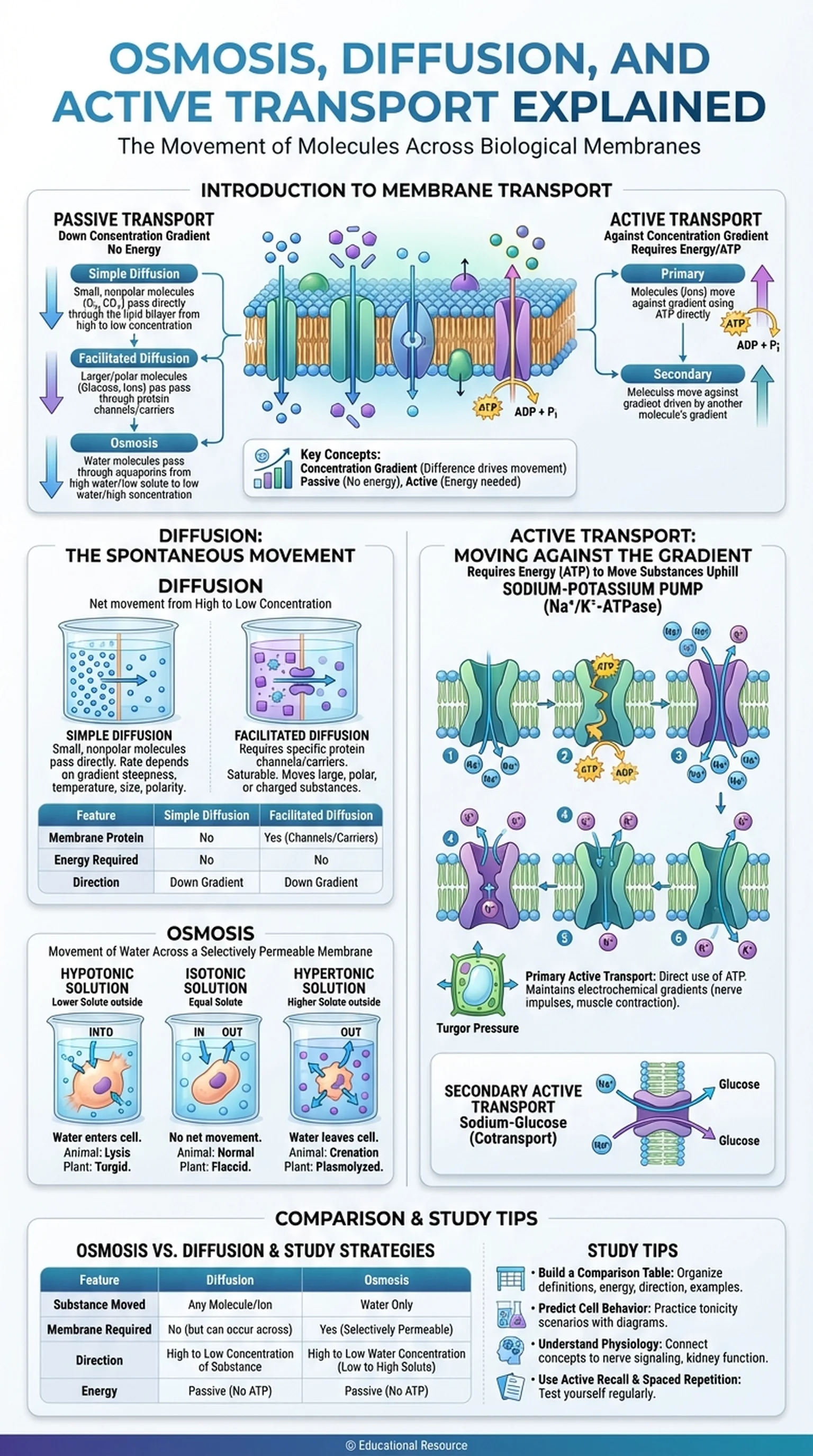
Introduction to Membrane Transport
The movement of molecules across biological membranes is one of the most fundamental processes in cell biology. Every living cell must import nutrients, export waste products, and maintain specific internal concentrations of ions and molecules to survive. These tasks are accomplished through several distinct transport mechanisms, the most important of which are diffusion, osmosis, and active transport. Understanding these processes is essential for students of biology, medicine, and physiology.
Membrane transport mechanisms are broadly classified into two categories: passive transport and active transport. Passive transport includes simple diffusion, facilitated diffusion, and osmosis, all of which move substances down their concentration gradient without requiring cellular energy. Active transport, by contrast, moves substances against their concentration gradient and requires energy, typically in the form of ATP. This fundamental distinction between energy-independent and energy-dependent movement is the organizing principle for understanding how cells regulate their internal environment.
The cell membrane itself, with its phospholipid bilayer and embedded proteins, is the gatekeeper that determines which molecules can cross and by what mechanism. Small nonpolar molecules like oxygen and carbon dioxide pass through the lipid bilayer freely by simple diffusion. Larger or polar molecules require protein channels or carriers for facilitated diffusion. Water moves by osmosis through aquaporins. Ions and large molecules that must move against their gradient rely on active transport pumps. Together, these mechanisms ensure that cells maintain homeostasis, the stable internal conditions necessary for biochemical reactions and life itself.
Key Terms
The movement of molecules and ions across biological membranes through passive or active mechanisms.
The movement of substances across a membrane down their concentration gradient without the expenditure of cellular energy.
The movement of substances across a membrane against their concentration gradient, requiring energy input, typically from ATP.
The difference in concentration of a substance between two areas, which drives the direction of passive transport.