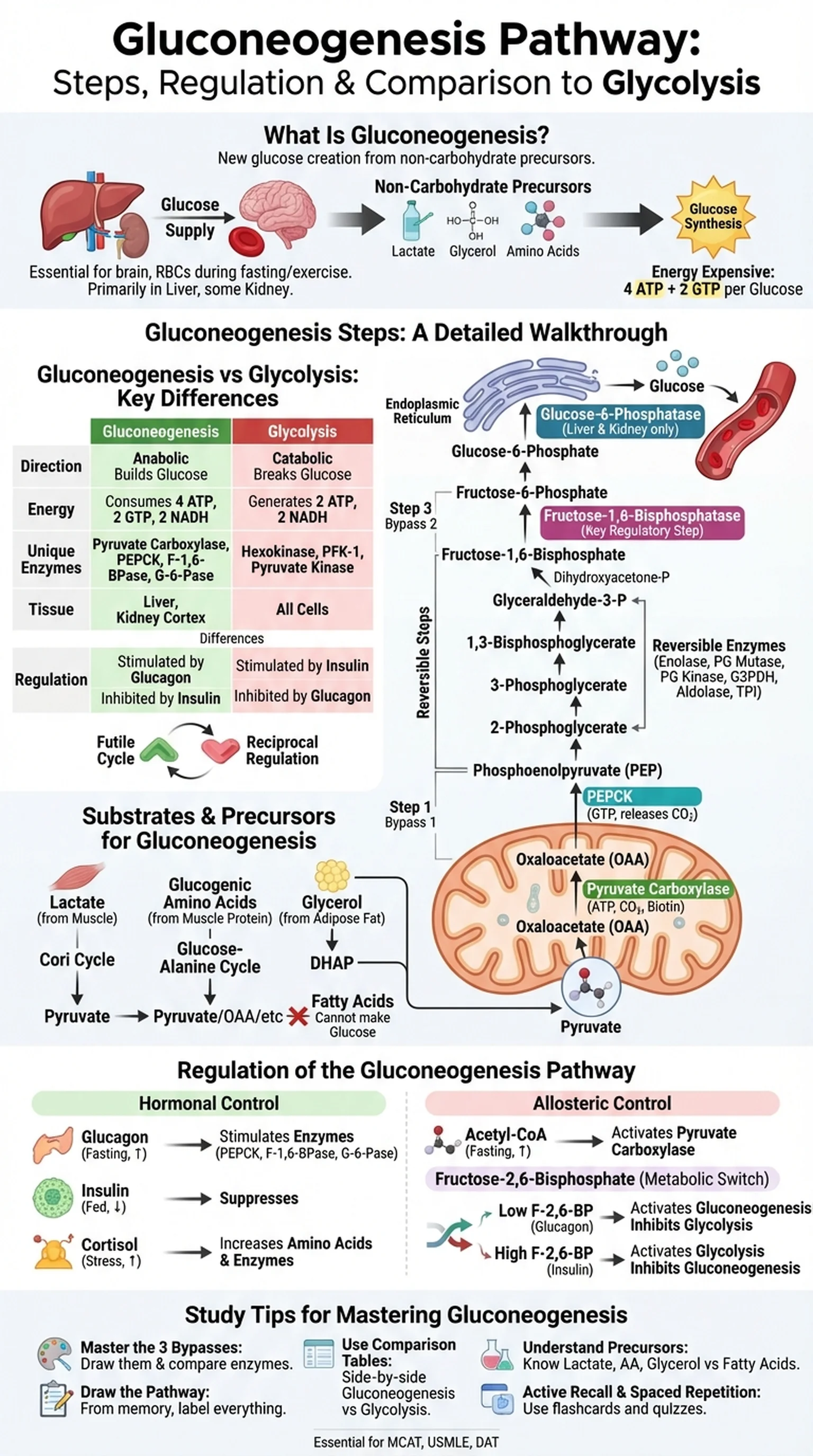
What Is Gluconeogenesis?
Gluconeogenesis is the metabolic pathway by which organisms synthesize glucose from non-carbohydrate precursors. The term itself is derived from Greek roots meaning "new glucose creation," and the pathway is essential for maintaining blood glucose levels during fasting, starvation, and intense exercise. Without gluconeogenesis, the brain, red blood cells, and renal medulla, all of which depend on glucose as their primary fuel, would be unable to function after glycogen stores are depleted.
The gluconeogenesis pathway occurs primarily in the liver, with a smaller contribution from the kidney cortex during prolonged fasting. It is essentially the reverse of glycolysis, converting pyruvate back into glucose through a series of enzymatic reactions. However, gluconeogenesis is not simply glycolysis running backward. Three steps of glycolysis are thermodynamically irreversible and must be bypassed by different enzymes unique to the gluconeogenesis pathway. These bypass reactions are catalyzed by pyruvate carboxylase, phosphoenolpyruvate carboxykinase (PEPCK), fructose-1,6-bisphosphatase, and glucose-6-phosphatase.
Glucose synthesis via gluconeogenesis is energetically expensive, requiring the input of six high-energy phosphate bonds (4 ATP + 2 GTP) to convert two molecules of pyruvate into one molecule of glucose. This energy cost underscores the biological importance of maintaining blood glucose: the body is willing to expend considerable ATP to ensure that glucose-dependent tissues receive a continuous supply. Understanding gluconeogenesis is fundamental for students studying biochemistry, physiology, and clinical medicine, as dysregulation of this pathway is central to the pathophysiology of type 2 diabetes and other metabolic diseases.
Key Terms
The metabolic pathway that synthesizes glucose from non-carbohydrate precursors such as pyruvate, lactate, glycerol, and glucogenic amino acids.
The production of new glucose molecules through the gluconeogenesis pathway, primarily in the liver and kidney cortex.
The series of enzymatic reactions that convert pyruvate to glucose, bypassing three irreversible steps of glycolysis with unique enzymes.
Molecules such as lactate, glycerol, and amino acids that can be converted into glucose via gluconeogenesis.